Technology
- HOME
- Technology
- Establishment of robust manufacturing process using QbD approach
Establishment of robust manufacturing process using QbD approach
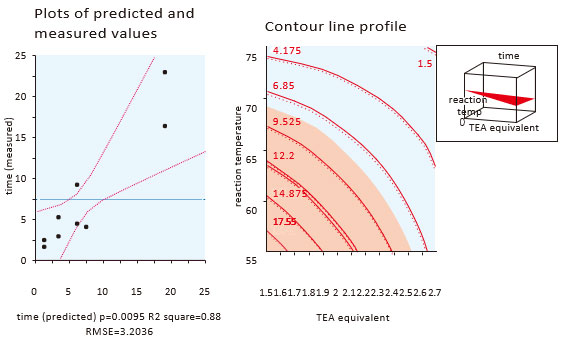
API process control based on ICH’s Q11 requires the process understanding in deep, by QbD approach as follows;
(1) selection of API’s critical quality attributes (CQAs) and risk assessment (FMEA), (2) extraction of PCPPs (Potential Critical Process Parameters) and evaluation by multi-dimensional experiment such as quality engineering and design of experiment (DoE) (3) identification of CPPs (Critical Process Parameters). The process optimization through the QbD approach provides a robust manufacturing process with focusing on the commercial application including responses to the inquiries from health authorities.
Inquiries About Service
SPERA provides CMC solutions from early-stage through new drug application.
Contact USpage top